Treatments for Breast Cancer in Postmenopausal Women
Sheryl Lawrence - Treatments for Breast Cancer in Postmenopausal Women
ABSTRACT
Breast Cancer is a disease that affects 264,000 women annually. Postmenopausal women over the age of fifty are at a higher risk of developing breast cancer because they are exposed to high levels of estrogen. Endocrine Therapy is supposed to inhibit hormone production and prevent hormones like estrogen from making breast cancer cells grow and divide. However, CDK 4/6 (proteins in the cell cycle) has a resistance to Endocrine Therapy therefore it allows breast cancer cell production to continue. This paper explores the various drugs (cdk4/6 inhibitor drugs, estrogen receptor degrader drugs, PI3k inhibitor drugs, mTOR inhibitor drugs, and SRC kinase inhibitor drugs) being assessed in clinical trials in the last 5 years to inhibit breast cancer cell production in postmenopausal women. The inclusion factors were if the article was a clinical study, written in the last 5 years (since 2018), was related to breast cancer in postmenopausal women. The exclusion criteria was if it did not include both“breast cancer”, and “postmenopausal women”. CDK 4/6 inhibitor drugs, estrogen receptor degrader drugs, PI3k inhibitor drugs, and mTor inhibitor drugs presented many benefits like increased life expectancy and reduction in breast cancer cells. SRC kinase drugs had no effect on breast cancer in postmenopausal women despite their many side effects. Certain drugs are better suited with certain types of breast cancer.
INTRODUCTION
Breast Cancer is a disease that affects 264,000 women annually [1]. Breast Cancer is when breast cancer tissue cells grow faster than normal which is caused by high levels of estrogen and progestin (hormones). Postmenopausal women over the age of fifty are at a higher risk of developing breast cancer because they are exposed to high levels of estrogen [2]. The cell cycle allows cells to replicate including cancer cells. Endocrine Therapy is supposed to inhibit hormone production and prevent hormones like estrogen from making breast cancer cells grow and divide [3]. However, CDK 4/6 (proteins in the cell cycle) has a resistance to Endocrine Therapy therefore it allows breast cancer cell production to continue [4]. Some drugs are inhibiting proteins in clinical studies: Ki67 is a protein associated with tumor aggressiveness, mTOR is associated with cell growth, and SRC kinase regulates cell growth.
The research question is, “what alternative drugs are being evaluated that can help inhibit Breast Cancer cell production?” This paper explores the various drugs (cdk4/6 inhibitor drugs, estrogen receptor degrader drugs, PI3k inhibitor drugs, mTOR inhibitor drugs, and SRC kinase inhibitor drugs) being assessed in clinical trials in the last 5 years to inhibit breast cancer cell production in postmenopausal women. All these drugs target specific proteins (CDK 4/6, Pi3K, mTOR, and SRC kinase) and protein receptors (estrogen receptors) which are being tested to determine if breast cancer cell production will diminish. Both the efficacy and side effects of each new treatment are discussed in the paper.
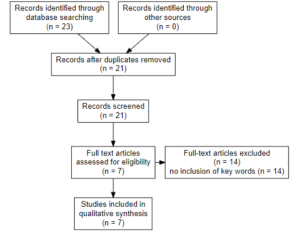
METHODS
Search Terms:
The search terms used were “Breast Cancer”, “Postmenopausal Women” and “Treatments.
Search Databases
I used the PUBMED database to gather all my information based on the search terms.
Inclusion and Exclusion Criteria
The inclusion factors were if the article was a clinical study, written in the last 5 years (since 2018), was related to breast cancer in postmenopausal women. The exclusion criteria was if it did not include both“breast cancer”, and “postmenopausal women”.
RESULTS
CDK 4/6 Inhibitor Drugs
A clinical study evaluated the change in Ki67 after treatment of abemaciclib, anastrozole, or abemaciclib plus anastrozole in Postmenopausal women with stage I-IIIB HR(+)/HER2(-) breast cancer. The change in Ki67 expression by demonstrating a greater decrease in expression of the tumor Ki67 levels after 2 weeks of abemaciclib alone (−91%) or in combination with anastrozole (−93%) compared with anastrozole alone (−63%) [5]. They also found that complete cell-cycle arrest was achieved in most patients after 2 weeks of treatment with abemaciclib either alone or in combination with anastrozole as compared with anastrozole alone [5]. Dr. Prat and Saura evaluated the impact of CDK4/6 inhibition with endocrine therapy in comparison to chemotherapy. The result is that, 23 (46·9%; 95% CI 32·5–61·7) of 49 patients in the ribociclib plus letrozole group and 24 (46·1%; 32·9–61·5) of 52 patients in the chemotherapy group were low-ROR (molecular degrading) [6]. CDK4/6 inhibitors combined with endocrine therapy had higher rates of neutropenia, leukopenia, thrombocytopenia, anemia, fatigue, diarrhea, febrile neutropenia, nausea and increased alanine aminotransferase (ALT) [7].
mTOR Inhibitor Drugs
Dr. Bardia and Modi evaluated a triple therapy of CDK4/6 inhibitor, mTOR inhibitor, and endocrine therapy. They had two study groups where one group received triple therapy while the other group did not. When examining tumor biopsies, they found that 14/33 patients treated with triplet therapy demonstrated a trend toward higher overall baseline expression of cell-cycle control genes and genes involved in the MAPK pathway in patients with PD compared with those with SD [8]. Dr. Angelo Leo and Dr. Stephen Johnston evaluated the effectiveness and safety of buparlisib plus fulvestrant in patients with advanced breast cancer who were pretreated with endocrine therapy and mTOR inhibitors. Between Jan 15, 2013, and March 31, 2016, 432 patients were randomly assigned to the buparlisib (n=289) or placebo (n=143) groups. Only 1- 2% of people experienced adverse side effects. Median progression-free survival was significantly longer in the buparlisib versus placebo group (3·9 months [95% CI 2·8–4·2] vs 1·8 months [1·5–2·8]; hazard ratio [HR] 0·67, 95% CI 0·53–0·84, one-sided p=0·00030) [9]. The side effects are hyperglycaemia, hypertension, and fatigue.
Estrogen receptor degrader
Dr. Bardia and Chandarlapaty evaluated once-daily amcenestrant, an oral drug that is a estrogen receptor (ER) degrader, in postmenopausal women with ER+/HER2- advanced breast cancer. The overall clinical benefit rate was 28.3% [10]. The drug revealed ER inhibition and degradation through paired tumor biopsies and cell-free DNA which showed detectable ERS1 mutations and Y537S mutations. The most frequently reported adverse events were as follows: hot flush (n = 5; 31.3%), diarrhea and nausea (n = 4 each; 25.0%), as well as decreased appetite, constipation, night sweats, and asthenia (n = 3 each; 18.8%) [11].
PI3K inhibitor
Dejan Juric and Janku evaluated PI3Kα-specific inhibitor (oral drug) plus fulvestrant in patients with ER+ advanced breast cancer (ABC). Alpelisib plus fulvestrant had a manageable safety profile with the alpelisib maximum tolerated dose of 400 mg and a recommended phase 2 dose of 300 mg once daily and objective response rate was higher (29% vs 0%) in patients with. Skin may be sensitive to combined PI3K inhibition with endocrine therapy resulting in rashes. However, they can be treated with the use of antihistamines, topical, or systemic corticosteroids [13].
SRC kinase inhibitor
Dr. Oswald and Symeonides evaluated the effect of saracatinib addition to aromatase inhibitors (AI) in patients with hormone receptor-positive metastatic breast cancer. Aracatinib was not associated with an improved PFS (3.7 months v. 5.6 months placebo/AI) and did not reduce likelihood of bony progression. Saracatinib was well tolerated with dose reductions in 16% and the main side effects were gastrointestinal, hypophosphatemia and rash. There was no observed beneficial effect on bone metastases [13].
DISCUSSION
While estrogen therapy was not effective for postmenopausal women with breast cancer, CDK 4/6 inhibitor drugs, estrogen receptor degrader drugs, pi3k inhibitor drugs, and mTor inhibitor drugs presented many benefits like increased life expectancy and reduction in breast cancer cells. SRC kinase drugs had no effect on breast cancer in postmenopausal women [13]. Triple therapy with endocrine therapy and mTOR and CDK4/6 inhibition is beneficial for postmenopausal women with HR+, HER2− ABC [11]. Overall, there are multiple newly tested drugs that are specifically beneficial for reducing breast cancer in postmenopausal women. Future research regarding breast cancer therapy in postmenopausal women should focus on creating a drug that reduces the amount of side effects such as anemia and nausea.
References
[1] Basic Information About Breast Cancer. (2023). Retrieved from CDC
[2] Cancer Causes and Prevention. (2015, April 29). Retrieved from National Cancer Institute
[3] Nathan-Garner, L. (2023). How does menopause affect cancer risk? Retrieved from MD Anderson Cancer Center
[4] Regulation of the Cell Cycle by External Events. (2023). LibreTexts Biology, 1.
[5] Hurvitz, S. A. (2020). Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR+/HER2− Breast Cancer. Clinical Cancer Research, 15.
[6] Hurvitz, S. A., Martin, M., Press, M. F., Chan, D., Fernandez-Abad, M., Petru, E., Rostorfer, R., Guarneri, V., Huang, C. S., Barriga, S., Wijayawardana, S., Brahmachary, M., Ebert, P. J., Hossain, A., Liu, J., Abel, A., Aggarwal, A., Jansen, V. M., & Slamon, D. J. (2020). Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR+/HER2- Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 26(3), 566–580. https://doi.org/10.1158/1078-0432.CCR-19-1425
[7] Prat, A., Saura, C., Pascual, T., Hernando, C., Muñoz, M., Paré, L., González Farré, B., Fernández, P. L., Galván, P., Chic, N., González Farré, X., Oliveira, M., Gil-Gil, M., Arumi, M., Ferrer, N., Montaño, A., Izarzugaza, Y., Llombart-Cussac, A., Bratos, R., González Santiago, S., … Gavilá, J. (2020). Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. The Lancet. Oncology, 21(1), 33–43. https://doi.org/10.1016/S1470-2045(19)30786-7
[8] Lin Yang, J. X. (2021). Side effects of CDK4/6 inhibitors in the treatment of HR+/HER2− advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Annals of Palliative Medicine, 14.
[9] Bardia, A., Modi, S., Oliveira, M., Cortes, J., Campone, M., Ma, B., Dirix, L., Weise, A., Hewes, B., Diaz-Padilla, I., Han, Y., Deshpande, P., Samant, T. S., Lorenc, K. R., He, W., Su, F., & Chavez-MacGregor, M. (2020). Phase Ib Dose-escalation/Expansion Trial of Ribociclib in Combination With Everolimus and Exemestane in Postmenopausal Women with HR+, HER2-
[10] Advanced Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 26(24), 6417–6428. https://doi.org/10.1158/1078-0432.CCR-20-1068
[11] Di Leo, A., Johnston, S., Lee, K. S., Ciruelos, E., Lønning, P. E., Janni, W., O’Regan, R., Mouret-Reynier, M. A., Kalev, D., Egle, D., Csőszi, T., Bordonaro, R., Decker, T., Tjan-Heijnen, V. C. G., Blau, S., Schirone, A., Weber, D., El-Hashimy, M., Dharan, B., Sellami, D., … Bachelot, T. (2018). Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. Oncology, 19(1), 87–100. https://doi.org/10.1016/S1470-2045(17)30688-5
[12] Bardia, A., Chandarlapaty, S., Linden, H. M., Ulaner, G. A., Gosselin, A., Cartot-Cotton, S., Cohen, P., Doroumian, S., Paux, G., Celanovic, M., Pelekanou, V., Ming, J. E., Ternès, N., Bouaboula, M., Lee, J. S., Bauchet, A. L., & Campone, M. (2022). AMEERA-1 phase 1/2 study of amcenestrant, SAR439859, in postmenopausal women with ER-positive/HER2-negative advanced breast cancer. Nature communications, 13(1), 4116. https://doi.org/10.1038/s41467-022-31668-8
[13] Juric, D., Janku, F., Rodón, J., Burris, H. A., Mayer, I. A., Schuler, M., Seggewiss-Bernhardt, R., Gil-Martin, M., Middleton, M. R., Baselga, J., Bootle, D., Demanse, D., Blumenstein, L., Schumacher, K., Huang, A., Quadt, C., & Rugo, H. S. (2019). Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA oncology, 5(2), e184475. https://doi.org/10.1001/jamaoncol.2018.4475
[14] Oswald, A. J., Symeonides, S. N., Wheatley, D., Chan, S., Brunt, A. M., McAdam, K., Schmid, P., Waters, S., Poole, C., Twelves, C., Perren, T., Bartlett, J., Piper, T., Chisholm, E. M., Welsh, M., Hill, R., Hopcroft, L. E. M., Barrett-Lee, P., & Cameron, D. A. (2023). Aromatase inhibition plus/minus Src inhibitor saracatinib (AZD0530) in advanced breast cancer therapy (ARISTACAT): a randomised phase II study. Breast cancer research and treatment, 199(1), 35–46. https://doi.org/10.1007/s10549-023-06873-8



