The Link Between Cancer Development And Environmental Pollutants In Impoverished Areas
ABSTRACT
This literature review aims to highlight the link between the development and progression of cancer and environmental pollution – especially in impoverished communities. The study utilized articles published within the past five years that reviewed the cancer incidences caused by environmental pollutants. This includes Particulate Matter 2.5 (PM2.5), asbestos, nitrates, waste incineration chemicals, petrochemical facilities, and what is a PFO, write it out like you did for PM2.5PFOs. For childhood PM2.5 exposure between the levels of 5.34 μg/m3 to 16.3 μg/m3, 58% of participants had an alteration in the TP53 gene, a tumor growth regulation gene. For asbestos exposure, approximately 15% of students had at least one type of primary cancer (where cancer originates growth in the body). In individuals who resided in close proximity to intensive animal agriculture practices, 519 incidences of lymphohematopoietic cancers were found. Individuals who lived near petrochemical facilities were more likely to develop Non-Hodgkin’s Lymphoma, with a relative risk of 1.06. The presence of Perfluorooctanoic acid (PFOA) and Perfluorooctane sulfonic acid (PFOS) led to an increased cell growth rate. In areas near waste incineration plants higher incidence of multiple myeloma, lung cancer, soft-tissue sarcoma, acute myeloid leukemia, myelodysplastic syndromes, and multiple myeloma were observed. Overall, a general positive correlation was indicated through the articles reviewed in this study between these chemicals and cancer development. This is evident by the development of cancer-causing genes, mutations in tumor regulatory genes, and the development of cancer drug resistance.
INTRODUCTION
According to the Centers for Disease Control (CDC), in 2021, cancer was the second leading cause of death after heart disease, with 605,213 cases reported [1]. Factors including genetics, lifestyle, and environmental conditions can influence cancer development and progression [2]. Harmful chemicals and pollutants like particulate matter (PM2.5), asbestos, nitrates, and Perfluorooctane (PFO) are just some of the handful explored in this literature review. PM2.5, a type of fine particulate matter, can cause epigenetic alterations that lead to lung cancer and activate tumor-associated signaling pathways [3]. Asbestos, and other silicate compounds, can lead to an increased risk of developing mesothelioma, enhanced when associated with para-occupational asbestos exposure prior to age 30 [4]. Nitrites and nitrates, compounds often found in foods and contaminated drinking water, have been shown to be associated with the development of stomach, brain, and colon cancer [5]. PFO can be associated with prostate, testicular, and kidney cancer. PFOs can be found in household items such as food, firefighting foam, and even water [6].
It is essential to note that many of these chemicals and pollutants are prominent in communities of low socioeconomic status (SES). According to the International Journal of Environmental Research and Public Health, cancer risk increases by 12-16% in low-income communities due to exposure to air toxins and other chemicals including benzene, formaldehyde, 1,3-butadiene, and naphthalene [7]. According to the National Cancer Institute, counties in the United States with consistently high poverty levels have a 12% higher death rate from cancer [5]. People living in low SES communities are at a higher risk of being exposed to carcinogens due to lower costs of living. The lower costs of living are often present in areas near carcinogen-producing plants and in old buildings that may have carcinogens in their building material [8]. This applies to surrounding areas of the community having high levels of carcinogens being polluted into the area, cheaper costs of foods that contain carcinogens, and cheaper housing in older buildings more prone to containing toxic carcinogens. This literature review aims to highlight the link between the development and progression of cancer and environmental pollution especially in impoverished communities.
METHODS
This paper reviews six papers describing various chemicals present in communities considered low SES, near chemical plants, petrochemical facilities, intensive animal agriculture, or the individuals whose occupation was located in those areas. Articles were excluded if they had no dates of studies, no fact sheets if the patients had multiple risk factors for cancer development, and if they were written more than five years ago. They were included for specifying the pollutant type and cancers it caused. In order to find more specific articles the keywords used in the search were narrowed down to specific pollutants like “PM2.5”, “asbestos”, “nitrates”, and “PFOs” in Google Scholar and PubMed. Specifying searches for “mesothelioma” also provided more suitable articles. By indicating residential proximity, articles were found that tied in the SES of the communities where cancers were found. The impoverished communities are defined by the poverty index specific to the nation. The review looks at the data on cancer rates in those areas, the types of cancers developed, the risk of cancer development, the presence of cancer-linked mutations, and the response to treatment presented in the reviewed studies.
RESULTS
One study originating in Denmark investigated the relationship between asbestos exposure and cancer risk for school children individuals living near an asbestos cement plant. The study utilized 12,111 children from schools in Aalborg, Denmark. Of the 12,111 students, 1,827 of the students had at least one type of primary cancer and 38 of them were malignant mesothelioma. This area was deemed to have the highest standardized incidence rate for asbestos-related cancers [9].
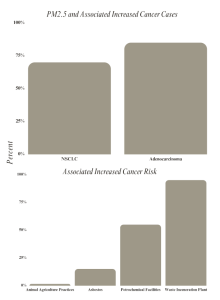
Another study analyzed cancer incidence in an area near a waste incineration plant. Waste incineration plants expose neighboring populations to atmospheric emissions and dioxin, a persistent organic pollutant. The study analyzed 80,865 cancer cases in the vicinity of a waste incineration plant in Nice, France between 2005 and 2014. The results indicated a higher incidence of lung cancer, soft-tissue sarcoma, acute myeloid leukemia, myelodysplastic syndromes, and multiple myeloma. The study also found a prevalence and persistence of multiple myeloma in people who lived in the area throughout the time period between 2010-2014, especially in men.[10].
Residential proximity to other facilities that produce carcinogenic materials was also analyzed. One study analyzed the residential proximity to intensive animal agriculture practices and cancer incidence. The study found 519 incidences of lymphohematopoietic cancers among pesticide applicators and 211 among spouses. There were 424 cases of Non-Hodgkin’s Lymphoma (NHL) among the pesticide applicators. The highest levels were found within 5km of weighted animal units. Almost all these participants lived near AFOs. This also coincided with an elevated NHL and B-Cell NHL risk [11].
Another study analyzed the hematological malignancies of individuals living near petrochemical facilities [12]. The study also analyzed NHL incidence in fenceline communities up to 7.5 kilometers from petrochemical facilities and the relative risk was 1.06 (95% CI = 0.97 to 1.17). The paper included a case study of a petrochemical corridor in Louisiana called “Cancer Alley” that had high levels of hazardous waste. According to the study, the facility was responsible for an average of 58.7 (CI = 27.8 to 105.1) new cases of leukemia in that area from 2011-2015. The study concluded to have a 30% higher risk of leukemia for residents living near petrochemical facilities [12].
Environmental pollutants were analyzed and it was found that low socioeconomic communities had higher concentrations of PM2.5 and higher odds of TP53 mutations, with 43% of patients having lived in moderate PM2.5 conditions as defined by the EPA. Among them, 58% had TP53 alterations identified through the analysis of somatic TP53 mutations. Among the tested tumor samples, 344 TP53 variants were identified in 277 patients. Of these mutations, 23% were located in pathogenic TP53 hotspot residues. Additionally, 40% of the identified variants were classified as loss-of-function or likely loss-of-function mutations. This alteration of TP53 was positively correlated with poor outcomes for patients with non-small cell lung cancer [13].
Lastly, I present a study that analyzed breast cancer progression and drug resistance after exposure to environmental chemicals. It was found that exposure to chemicals like dioxins, polycyclic aromatic hydrocarbons (PAHs), nicotine, toxic metals, and pesticides can create an epithelial-to-mesenchymal transition, which is critical in cancer development and resistance to chemotherapy. Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) were found to stimulate cell migration and invasion, suggesting their potential role in breast cancer progression. The presence of PFOA and PFOS led to increased cell growth rate, promoting cell proliferation by accelerating the transition from G0/G1 to S phase in the cell cycle. This caused increased levels of cyclin D1, CDK4/6, and CDK4, as well as decreased levels of p27, p21, and p53 [14].
DISCUSSION
Many of the studies found an increased expression of tumor-causing genes in patients with high exposure to environmental pollutants [13,14]. This opens the door for those of low SES to have a higher probability of cancer risk in the future. Depending on the chemical and how individuals were exposed to that chemical, different genes and signaling pathways were impacted. The presence of cancer in the lymph nodes is alarming as it indicates the presence of cancer in other parts of the body or future metastasis. One study was also associated with higher levels of drug resistance [14]. This is especially alarming as it decreases the chances of a positive prognosis. The findings of the reviewed studies call for the mitigation of the presence of carcinogenic materials like asbestos especially in occupational, environmental, and household settings. This also applies to pollutants like PM 2.5 and AFOs. When analyzing the AFO exposure intensity, it was found that areas with higher AFO exposure had higher percentages of low SES communities in comparison to low-exposure areas. The town of Nice is shown to have major socioeconomic disparities with 10% of the population living below the poverty line [10]. High levels of poverty and illiteracy were observed in one study which can be extremely harmful in educating patients on the harms of these carcinogens and the importance of preventive care [12]. This warrants further investigation of individuals living near carcinogenic producing facilities and calls for public health policies, preventive strategies, and regulatory standards [12].
Some limitations of this literature review involve the varying definitions of what it means to be considered low SES. The varying countries and their varying access to healthcare and early prevention may have also impacted the results and interoperations of this review. The review supports the conclusion that environmental pollutants increase the chances of developing cancer, especially in low socioeconomic areas. It was also shown that low socioeconomic communities have a decreased likelihood of seeking preventive care [16]. This, along with heightened exposure to cancer-causing chemicals exacerbates their conditions. The decreased likelihood of seeking preventive care varies depending on country and circumstance however, it generally is due to lack of patient education or the costs of healthcare [16]. It is important that change is made to create safer neighborhoods for all individuals. This includes potential bans or limitations on cancer-causing chemicals. It is also vital that changes are made to the costs of healthcare allowing patients to get regular screenings and checkups. Potential alternatives for this may include making medical screenings and checkups more accessible and increasing patient education on the vitality of preventive checkups and signs and symptoms to look out for.
References
- CDC. (2023, January 18). Leading Causes of Death. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
- Institute of Medicine (US) Roundtable on Environmental Health Sciences, R., Wilson, S., Jones, L., Couseens, C., & Hanna, K. (2002). The Links Between Environmental Factors, Genetics, and the Development of Cancer. In www.ncbi.nlm.nih.gov. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK220897/
- Li, R., Zhou, R., & Zhang, J. (2018). Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases (Review). Oncology Letters, 15(5). https://doi.org/10.3892/ol.2018.8355
- Noonan, C. W. (2017). Environmental asbestos exposure and risk of mesothelioma. Annals of Translational Medicine, 5(11), 234–234. https://doi.org/10.21037/atm.2017.03.74
- National Cancer Institute. (2022, September 15). Persistent Poverty and Cancer – NCI. Www.cancer.gov. https://www.cancer.gov/research/annual-plan/scientific-topics/increasing-health-equity#:~:text=Recent%20research%20has%20shown%20that
- Essien, E. E., Said Abasse, K., Côté, A., Mohamed, K. S., Baig, M. M. F. A., Habib, M., Naveed, M., Yu, X., Xie, W., Jinfang, S., & Abbas, M. (2020). Drinking-water nitrate and cancer risk: A systematic review and meta-analysis. Archives of Environmental & Occupational Health, 77(1), 51–67. https://doi.org/10.1080/19338244.2020.1842313
- James, W., Jia, C., & Kedia, S. (2012). Uneven Magnitude of Disparities in Cancer Risks from Air Toxics. International Journal of Environmental Research and Public Health, 9(12), 4365–4385. https://doi.org/10.3390/ijerph9124365
- Larsen, K., Rydz, E., & Peters, C. E. (2023). Inequalities in Environmental Cancer Risk and Carcinogen Exposures: A Scoping Review. International Journal of Environmental Research and Public Health, 20(9), 5718. https://doi.org/10.3390/ijerph20095718
- Dalsgaard, S. B., Würtz, E. T., Hansen, J., Røe, O. D., & Omland, Ø. (2021). Cancer Incidence and Risk of Multiple Cancers after Environmental Asbestos Exposure in Childhood—A Long-Term Register-Based Cohort Study. International Journal of Environmental Research and Public Health, 19(1), 268. https://doi.org/10.3390/ijerph19010268
- Mariné Barjoan, E., Doulet, N., Chaarana, A., Festraëts, J., Viot, A., Ambrosetti, D., Lasalle, J.-L., Mounier, N., Bailly, L., & Pradier, C. (2020). Cancer incidence in the vicinity of a waste incineration plant in the Nice area between 2005 and 2014. Environmental Research, 188, 109681. https://doi.org/10.1016/j.envres.2020.109681
- Fisher, J. A., Freeman, L. E. B., Hofmann, J. N., Blair, A., Parks, C. G., Thorne, P. S., Ward, M. H., & Jones, R. R. (2020). Residential Proximity to Intensive Animal Agriculture and Risk of Lymphohematopoietic Cancers in the Agricultural Health Study. Epidemiology, 31(4), 478–489. https://doi.org/10.1097/ede.0000000000001186
- Jephcote, C., Brown, D., Verbeek, T., & Mah, A. (2020). A systematic review and meta-analysis of haematological malignancies in residents living near petrochemical facilities. Environmental Health, 19(1). https://doi.org/10.1186/s12940-020-00582-1
- Erhunmwunsee, L., Wing, S. E., Shen, J., Hu, H., Sosa, E., Lopez, L. N., Raquel, C., Sur, M., Ibarra-Noriega, P., Currey, M., Lee, J., Kim, J. Y., Raz, D. J., Amini, A., Sampath, S., Koczywas, M., Massarelli, E., West, H. L., Reckamp, K. L., & Kittles, R. A. (2021). The Association between Polluted Neighborhoods and TP53-Mutated Non-Small Cell Lung Cancer. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 30(8), 1498–1505. https://doi.org/10.1158/1055-9965.EPI-20-1555
- Koual, M., Tomkiewicz, C., Cano-Sancho, G., Antignac, J.-P., Bats, A.-S., & Coumoul, X. (2020). Environmental chemicals, breast cancer progression and drug resistance. Environmental Health, 19, 117. https://doi.org/10.1186/s12940-020-00670-2
- Steenland, K., & Winquist, A. (2021). PFAS and cancer, a scoping review of the epidemiologic evidence. Environmental Research, 194, 110690. https://doi.org/10.1016/j.envres.2020.110690
- Fleary, S. A., Ettienne-Gittens, R., & Heffer, R. W. (2013). Perceptions of Preventive Health Care and Healthy Lifestyle Choices for Low Income Families: A Qualitative Study. ISRN Preventive Medicine, 2013, 1–6. https://doi.org/10.5402/2013/189180



